The solvent was removed using a rotary evaporator. In this experiment caffeine was isolated from tealeaves through extraction distillation and sublimation.
Caffeine Isolation From Tea Bags
The ideal solvent in the extraction should have a low boiling point not react with the solute or other solvents not be toxic or highly flammable not miscible with.
. This is essentially the same procedure used to decaffeinate drinks such as coffee and tea. 125 mg in a typical 150 mL cup serving. Bring in two tea bags from home.
4075 or 053 g of caffeine in 14 g of tea leaves. Add 20 ml dichloromethane and 10 ml 02 M NaOH. Although chlorophylls are soluble in methylene chloride and ethyl acetate most other substances in tea are not.
The amount of caffeine in tea varies by brand but the average in common brands is typically about 30-40 mg caffeine tea bag. In the first step we have to brew a very strong cup of tea. Use an organic solvent to extract the caffeine and related compounds from the water.
If you recovered 04 g of caffeine from the 150 mL of tea following extraction this means that the total amount of caffeine in the 14 g of tea leaves is. 05314 100 38 by weight of caffeine in tea. Bring about 100 ml of water to boil in a 150 ml beaker.
Anhydrous calcium chloride pellets were used to dry the solution and. In an experiment using 2 tea bags 110 mg is the expected yield of caffeine to obtain. The remaining liquid was lost in the tea leaves and in the process of filtering.
Take 500 ml beaker add 200 ml of distilled water to it. Place the tea bags in the boiling water and let them steep for 7 - 10 minutes with a watch glass. The approximate weight of an individual Lipton tea bag is 200 005 g containing 55 mg of caffeine per bag.
Extraction and Recrystallization of Cafeine from Tea Name. Use hot water to extract the caffeine from the tea leaves. Place the tea leaves in a 125-ml Erlenmeyer flask.
The purpose of this experiment was to perform a liquid-liquid extraction method to extract the caffeine from the tea bags that were provided and then recrystallize the caffeine. Cafein e weight g 00124 3. Open the tea bags and weigh the contents.
1 Isolation of caffeine 6 tea bags 5 g of calcium carbonate powder and 180 mlshow more content. This data may be imprecise because during the extraction of caffeine emulsions may have occurred. Extraction of Caffeine 20ml of 10 sulphuric acid was added to the filtrate and stirred.
Because of the presence of Caffeine tea and coffee are gaining popularity as an addictive stimulant. The solvents used in the experiment were an aqueous sodium carbonate and dichloromethane DCM. Seal the flask and gently swirl it for 5-10 minutes to allow the solvent mixture to penetrate the leaves.
Extraction of Caffeine from the Tea Solution Pour the tea solution into a 60 mL separatory funnel be sure that the stopcock is closed before you add the tea solution to the separatory funnel. Organic Chem Laboratory Manual 35 Rating. This will help you determine how well your procedure worked.
Extract the tea solution with 6 mL of dichloromethane CH 2 Cl. Extraction of Caffeine. In the case of Caffeine extraction from tea powder the solubility of caffeine in water is 22mgml at.
Tea bags are used as the source of caffeine for this experiment. Properties synthesis and isolation from tea. A 50 mL beaker along with 2 boiling stones was weighed in advance with a total mass of 2756 g during the extraction process.
Vuong QV Bowyer MC and Roach PD. The reaction involves a homogenous mixture of an organic and aqueous layer. The intention of this lab was to learn how to isolate caffeine from tea leaves using the method of extraction.
Caffeine Lab 1 Isolation of Caffeine from tea In this experiment caffeine will be extracted from tea leaves where it is about 5 present using hot water. Sulphuric acid converts the tannins to their salts therefore making them insoluble in chloroform though soluble in water. Caffeine also known as Guaranine Methyltheobromine and Thein is defined as a naturally occurring chemical stimulant of the central nervous system that occurs in beans leaves fruit and beverages such as tea coffee and soda.
The brown color of a tea solution is due to the flavenoid pigments and chlorophylls and to their respective oxidation products. An extraction is taking place each time coffee or tea is made. Tea 30-75 mg per cup Cocoa 5-40 mg per cup Milk chocolate 6 mg per ounce Baking chocolate 35 mg per ounce Coca-Cola 46 mg per 12 ounces Excedrin extra strength 65 mg per tablet No-Doz 100 mg per tablet Experiment 6 Isolation of Caffeine from Tea Leaves Introduction Caffeine is a member of the class of compounds organic chemists call.
Add about 4 g of sodium carbonate. The obtained mass of caffeine was measured. While cellulose is insoluble in the water the tannins and chlorophyll will extract along with the caffeine into the water.
Thus the extraction of the basic tea solution with either solvent removes nearly pure caffeine. ISOLATION OF CAFFEINE FROM TEA EXPERIMENTAL TECHNIQUES REQUIRED Extraction T 6 drying agents T 7 filtration T 3 rotary evaporation T 8 recrystallisation T 2 andor. Of-white shape powdery Description Recrystallized Cafeine.
The percentage yield based on the amount of tea originally used was calculated. The melting point was estimated. Procedure Add about 100 g of tea leaves to around 100 mL of water in a 400-mL beaker cut open.
The solubility of caffeine is 22 mgmL 25 C and 670 mgmL 100 C. Caffeine extraction from tea leaves involves an acidbase liquidliquid extraction Oneota 2003. Experiment 2 Isolation and Sublimation of Caffeine from Tea Leaves Reading Assignment Mohrig Chapter 10 extraction intro to Chapter 16 sublimation Extraction is the physical process by which a compound or mixture of compounds is transferred from one phase to another.
Six teabags were steeped for. In this experiment we will isolate caffeine from tea. Cafein e weight g 00184 2.
This happens when an organic compound of one liquid in a. An average 30g of tea can contain 20-ll0 mg of caffeine thereby making tea a significant source of caffeine compared to other beverages. Now place the 5 tea bags in this beaker.
Wu Jennifer CHE 2403 Signature Lab Series Page 65-73. Description Crude Cafeine Crystals. Isolation of Caffeine from Tea Hayley Williams willi553gostocktonedu CHEM 2125-007 March 20 2018 Abstract.
Isolating Caffeine from Tea page 65-73 Signature Lab Series CHE 2403 Cengage Learning. Take 5 tea bags and record the weight of these tea bags.
Ppt Isolation Of Caffeine From Tea Leaves Powerpoint Presentation Free Download Id 1607508
Isolation And Characterization Of Caffeine From Waste Tea Semantic Scholar
Extraction Procedure For The Catechins And Caffeine Analysis From The Download Scientific Diagram
Solved Experiment 2 Isolation Of Caffeine From Tea Aims Chegg Com
Lab Report Extraction Of Caffeine From Tea Lab Report Experiment Extraction Of Caffeine From Studocu
Pdf Isolation And Characterization Of Caffeine From Waste Tea
Doc Isolation Of Caffeine From Tea Elizabeth Ping Academia Edu
Extraction Of Caffeine From Tea Leaves
Experiment Isolation Of Caffeine From Tea Leaves Youtube
Experiment 2 Isolation Of Caffeine From Tea Leaves
Extraction And Isolation Of Caffeine From Tea Leaves English By Solution Pharmacy Youtube
Aldawaa Isolation Of Caffeine From Tea
How To Extract Caffeine From Tea Classic Dcm Method Youtube
Pdf Experiment 2 Isolation And Sublimation Of Caffeine From Tea Leaves 성철 홍 Academia Edu
Extraction Isolation Of Caffeine From Tea
One Part Of Chemistry Extraction Of Caffeine From Tea Leaves
Lecture 3 A Extraction Of Caffeine From Tea
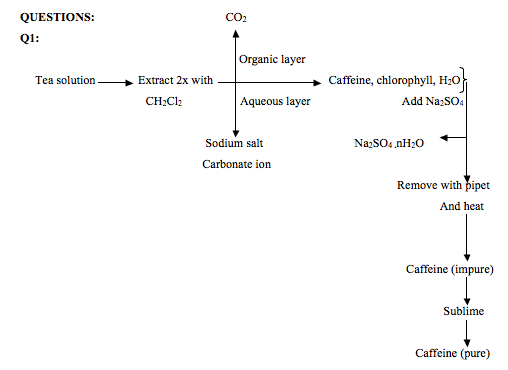
Theoutline Of A Separation Scheme For Isolating Caffeine From Tea Needs To Be Explained Concept Introduction In The Isolation Of Caffeine From Tea Leaves The Main Problem Is That The Caffeine Is
Ppt Isolation Of Caffeine From Tea Leaves Powerpoint Presentation Free Download Id 1607508
